Law Of Multiple Proportions Explained
4.3: Law of Multiple Proportions
- Folio ID
- 52750
What are the similarities and differences between a unicycle and a bicycle?
Just from the words themselves, the astute Latin-speaking scholar tin tell that, any it is made of, the unicycle has one of them (uni = "one") and the bicycle has two (bi = "two"). From pictures, nosotros get boosted information that helps usa tell the two apart. The unicycle has i wheel and the bicycle has two. In particular, they are made up of the same materials, and the simply pregnant difference is the number of wheels on the two vehicles. At present—how many wheels are on a tricycle?
Police force of Multiple Proportions
Once the idea that elements combined in definite proportions to form compounds was established, experiments likewise began to demonstrate that the same pairs of certain elements could combine to form more than than one chemical compound. Consider the elements carbon and oxygen. Combined in one way, they class the familiar compound carbon dioxide. In every sample of carbon dioxide, there are \(32.0 \: \text{g}\) of oxygen present for every \(12.0 \: \text{g}\) of carbon. By dividing \(32.0\) by \(12.0\), this simplifies to a mass ratio of oxygen to carbon of 2.66 to 1. There is another compound that forms from the combination of carbon and oxygen called carbon monoxide. Every sample of carbon monoxide contains \(16.0 \: \text{chiliad}\) of oxygen for every \(12.0 \: \text{yard}\) of carbon. This is a mass ratio of oxygen to carbon of 1.33 to 1. In the carbon dioxide, there is exactly twice as much oxygen present as in that location is in the carbon monoxide. This example illustrates the law of multiple proportions: whenever the same two elements form more one compound, the different masses of 1 element that combine with the same mass of the other element are in the ratio of small whole numbers.
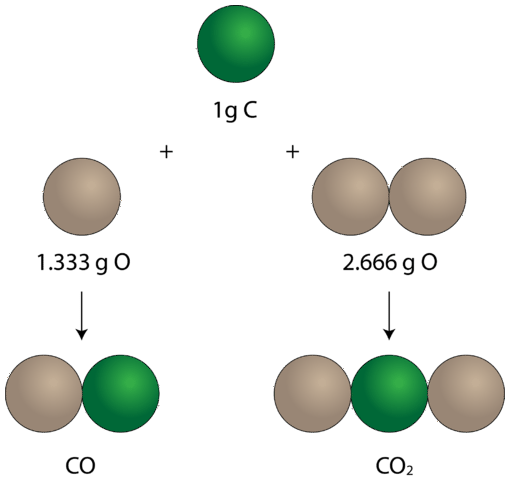
In carbon monoxide, on the left, there is \(1.333 \: \text{thousand}\) of oxygen for every \(ane \: \text{yard}\) of carbon. In carbon dioxide, on the right, there is \(two.666 \: \text{g}\) of oxygen for every gram of carbon. And so the ratio of oxygen in the two compounds is 1:two, a small whole number ratio.
The difference between carbon monoxide and carbon dioxide is pregnant. Carbon monoxide is a deadly gas, formed from the incomplete combustion of some carbon-containing materials (such every bit woods and gasoline). This compound will attach to hemoglobin in the red claret cells and block the bounden of oxygen to those cells. If oxygen does not bind, it cannot be carried to the cells of the torso where it is needed, and death can occur. Carbon dioxide, on the other hand, is non toxic similar carbon monoxide is. However, information technology can readapt oxygen in systems since it is heavier. Carbon dioxide fire extinguishers cut off the flow of oxygen in a fire, putting out the fire.
Summary
- The constabulary of multiple proportions states that whenever the aforementioned 2 elements form more than one compound, the unlike masses of i element that combine with the same mass of the other element are in the ratio of small whole numbers.
Review
- State the law of multiple proportions.
- In carbon dioxide (CO2), how many grams of oxygen (O) would in that location be if in that location are 24 grams of carbon (C)?
- How many grams of carbon (C) would be nowadays in carbon monoxide (CO) that contains two.666 grams of oxygen (O)?
Law Of Multiple Proportions Explained,
Source: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/04%3A_Atomic_Structure/4.03%3A_Law_of_Multiple_Proportions
Posted by: richardssichiple.blogspot.com


0 Response to "Law Of Multiple Proportions Explained"
Post a Comment