Beer's Law Y Mx B
1.ii: Beer's Police
- Page ID
- 111324
What factors influence the absorbance that you would measure out for a sample? Is each factor directly or inversely proportional to the absorbance?
One factor that influences the absorbance of a sample is the concentration (c). The expectation would exist that, every bit the concentration goes up, more radiation is absorbed and the absorbance goes up. Therefore, the absorbance is directly proportional to the concentration.
A 2nd gene is the path length (b). The longer the path length, the more than molecules in that location are in the path of the beam of radiation, therefore the absorbance goes upwards. Therefore, the path length is directly proportional to the concentration.
When the concentration is reported in moles/liter and the path length is reported in centimeters, the third factor is known as the molar absorptivity (\(\varepsilon\)). In some fields of piece of work, it is more common to refer to this as the extinction coefficient. When we apply a spectroscopic method to measure the concentration of a sample, nosotros select out a specific wavelength of radiation to shine on the sample. Equally you lot probable know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not others. The molar absorptivity is a measure of how well the species absorbs the particular wavelength of radiation that is being shined on it. The process of absorbance of electromagnetic radiation involves the excitation of a species from the ground state to a higher energy excited state. This procedure is described as an excitation transition, and excitation transitions have probabilities of occurrences. It is advisable to talk about the degree to which possible energy transitions within a chemic species are allowed. Some transitions are more than allowed, or more favorable, than others. Transitions that are highly favorable or highly allowed have high molar absorptivities. Transitions that are only slightly favorable or slightly allowed have low molar absorptivities. The higher the tooth absorptivity, the higher the absorbance. Therefore, the molar absorptivity is directly proportional to the absorbance.
If we render to the experiment in which a spectrum (recording the absorbance every bit a part of wavelength) is recorded for a compound for the purpose of identification, the concentration and path length are abiding at every wavelength of the spectrum. The only difference is the tooth absorptivities at the different wavelengths, then a spectrum represents a plot of the relative molar absorptivity of a species as a function of wavelength.
Since the concentration, path length and molar absorptivity are all straight proportional to the absorbance, we can write the following equation, which is known every bit the Beer-Lambert law (oftentimes referred to as Beer's Constabulary), to show this relationship.
\[\mathrm{A = \varepsilon bc} \nonumber \]
Note that Beer'south Law is the equation for a straight line with a y-intercept of zero.
If you lot wanted to mensurate the concentration of a item species in a sample, describe the procedure you would utilise to do so.
Measuring the concentration of a species in a sample involves a multistep procedure.
Ane important consideration is the wavelength of radiation to use for the measurement. Remember that the college the molar absorptivity, the higher the absorbance. What this also means is that the higher the molar absorptivity, the lower the concentration of species that still gives a measurable absorbance value. Therefore, the wavelength that has the highest molar absorptivity (\(\lambda\)max) is usually selected for the analysis because information technology volition provide the lowest detection limits. If the species you are measuring is 1 that has been commonly studied, literature reports or standard assay methods will provide the \(\lambda\)max value. If it is a new species with an unknown \(\lambda\)max value, then it is easily measured by recording the spectrum of the species. The wavelength that has the highest absorbance in the spectrum is \(\lambda\)max.
The second step of the process is to generate a standard bend. The standard curve is generated by preparing a series of solutions (unremarkably 3-v) with known concentrations of the species existence measured. Every standard curve is generated using a blank. The blank is some appropriate solution that is causeless to accept an absorbance value of zero. It is used to nothing the spectrophotometer before measuring the absorbance of the standard and unknown solutions. The absorbance of each standard sample at \(\lambda\)max is measured and plotted every bit a function of concentration. The plot of the data should be linear and should go through the origin equally shown in the standard curve in Figure \(\PageIndex{two}\). If the plot is not linear or if the y-intercept deviates substantially from the origin, it indicates that the standards were improperly prepared, the samples deviate in some manner from Beer'south Police force, or that there is an unknown interference in the sample that is complicating the measurements. Assuming a linear standard curve is obtained, the equation that provides the best linear fit to the data is generated.

Notation that the slope of the line of the standard curve in Effigy \(\PageIndex{2}\) is (\(\varepsilon\)b) in the Beer'due south Constabulary equation. If the path length is known, the slope of the line can so be used to summate the molar absorptivity.
The third pace is to measure the absorbance in the sample with an unknown concentration. The absorbance of the sample is used with the equation for the standard curve to calculate the concentration.
Suppose a small amount of stray radiation (PDue south) always leaked into your musical instrument and fabricated information technology to your detector. This stray radiation would add to your measurements of Po and P. Would this cause whatsoever deviations to Beer's police force? Explain.
The way to call back nearly this question is to consider the expression we wrote before for the absorbance.
\[\mathrm{A = \log\left(\dfrac{P_o}{P}\right)} \nonumber \]
Since devious radiation always leaks in to the detector and presumably is a fixed or constant quantity, we can rewrite the expression for the absorbance including terms for the stray radiation. It is important to recognize that Po, the power from the radiation source, is considerably larger than \(P_S\). Also, the numerator (Po + Ps) is a constant at a detail wavelength.
\[\mathrm{A = \log\left(\dfrac{P_o + P_s}{P + P_s}\correct)} \nonumber \]
Now let'south examine what happens to this expression nether the two extremes of low concentration and high concentration. At low concentration, non much of the radiation is absorbed and P is not that much different than Po. Since \(P_o \gg P_S\), \(P\) will also exist much greater than \(P_S\). If the sample is at present fabricated a piddling more concentrated so that a little more than of the radiation is captivated, P is still much greater than PS. Under these weather the amount of devious radiation is a negligible contribution to the measurements of Po and P and has a negligible effect on the linearity of Beer's Law.
As the concentration is raised, P, the radiations reaching the detector, becomes smaller. If the concentration is made high enough, much of the incident radiation is absorbed by the sample and P becomes much smaller. If we consider the denominator (P + PS) at increasing concentrations, P gets minor and PS remains constant. At its limit, the denominator approaches PDue south, a constant. Since Po + PS is a constant and the denominator approaches a constant (Psouth), the absorbance approaches a abiding. A plot of what would occur is shown in Figure \(\PageIndex{3}\).

The ideal plot is the directly line. The curvature that occurs at higher concentrations that is caused by the presence of devious radiation represents a negative deviation from Beer's Law.
The derivation of Beer's Law assumes that the molecules arresting radiations don't interact with each other (remember that these molecules are dissolved in a solvent). If the analyte molecules interact with each other, they can alter their ability to absorb the radiation. Where would this assumption break down? Estimate what this does to Beer'southward constabulary?
The sample molecules are more probable to interact with each other at college concentrations, thus the assumption used to derive Beer'southward Police force breaks down at high concentrations. The event, which we will non explain in whatsoever more detail in this document, too leads to a negative deviation from Beer'due south Law at loftier concentration.
Beer's law also assumes purely monochromatic radiation. Draw an instrumental fix that would permit you lot to polish monochromatic radiation on your sample. Is it possible to get purely monochromatic radiations using your set? Guess what this does to Beer'southward law.
Spectroscopic instruments typically have a device known as a monochromator. There are two cardinal features of a monochromator. The outset is a device to disperse the radiation into distinct wavelengths. You lot are likely familiar with the dispersion of radiation that occurs when radiation of unlike wavelengths is passed through a prism. The second is a slit that blocks the wavelengths that you lot exercise not want to shine on your sample and simply allows \(\lambda\)max to pass through to your sample equally shown in Figure \(\PageIndex{4}\).
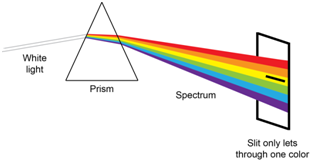
An examination of Figure \(\PageIndex{4}\) shows that the slit has to let some "packet" of wavelengths through to the sample. The bundle is centered on \(\lambda\)max, but clearly nearby wavelengths of radiations pass through the slit to the sample. The term effective bandwidth defines the packet of wavelengths and information technology depends on the slit width and the ability of the dispersing element to dissever the wavelengths. Reducing the width of the slit reduces the packet of wavelengths that make it through to the sample, significant that smaller slit widths atomic number 82 to more monochromatic radiations and less deviation from linearity from Beer's Constabulary.
Is there a disadvantage to reducing the slit width?
The important thing to consider is the upshot that this has on the ability of radiation making it through to the sample (Po). Reducing the slit width will lead to a reduction in Po and hence P. An electronic measuring device called a detector is used to monitor the magnitude of Po and P. All electronic devices have a groundwork noise associated with them (rather analogous to the static racket you may hear on a speaker and to the discussion of stray radiations from before that represents a form of dissonance). Po and P represent measurements of betoken over the background noise. Every bit Po and P become smaller, the groundwork dissonance becomes a more significant contribution to the overall measurement. Ultimately the background racket restricts the indicate that tin be measured and detection limit of the spectrophotometer. Therefore, it is desirable to accept a large value of Po. Since reducing the slit width reduces the value of Po, it also reduces the detection limit of the device. Selecting the appropriate slit width for a spectrophotometer is therefore a balance or tradeoff of the want for loftier source power and the desire for high monochromaticity of the radiation.
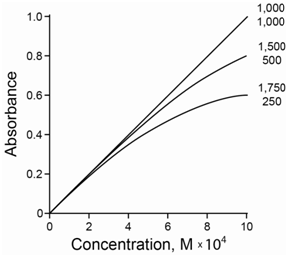
Information technology is not possible to get purely monochromatic radiation using a dispersing element with a slit. Commonly the sample has a slightly different molar absorptivity for each wavelength of radiation shining on it. The cyberspace effect is that the total absorbance added over all the different wavelengths is no longer linear with concentration. Instead a negative divergence occurs at higher concentrations due to the polychromicity of the radiation. Furthermore, the deviation is more pronounced the greater the difference in the molar absorbtivity. Figure \(\PageIndex{5}\) compares the deviation for two wavelengths of radiations with tooth absorptivities that are (a) both 1,000, (b) 500 and 1,500, and (c) 250 and 1,750. As the molar absorptivities become further apart, a greater negative divergence is observed.
Therefore, it is preferable to perform the absorbance measurement in a region of the spectrum that is relatively broad and flat. The hypothetical spectrum in Figure \(\PageIndex{6}\) shows a species with ii wavelengths that have the same molar absorptivity. The peak at approximately 250 nm is quite sharp whereas the ane at 330 nm is rather broad. Given such a choice, the broader peak will have less deviation from the polychromaticity of the radiations and is less prone to errors caused by slight misadjustments of the monochromator.

Consider the relative mistake that would exist observed for a sample as a part of the transmittance or absorbance. Is in that location a preferable region in which to mensurate the absorbance? What practice you think about measuring absorbance values above 1?
It is important to consider the error that occurs at the two extremes (high concentration and depression concentration). Our discussion above near deviations to Beer'southward Law showed that several problems ensued at college concentrations of the sample. Also, the point where only 10% of the radiations is transmitted through the sample corresponds to an absorbance value of 1. Because of the logarithmic human relationship between absorbance and transmittance, the absorbance values rising rather rapidly over the last x% of the radiation that is absorbed by the sample. A relatively small alter in the transmittance can lead to a rather large change in the absorbance at loftier concentrations. Because of the substantial negative deviation to Beer's police force and the lack of precision in measuring absorbance values above ane, it is reasonable to presume that the fault in the measurement of absorbance would be high at loftier concentrations.
At very low sample concentrations, we observe that Po and P are quite similar in magnitude. If we lower the concentration a bit more, P becomes even more like to Po. The important realization is that, at low concentrations, we are measuring a small difference between 2 big numbers. For example, suppose we wanted to measure out the weight of a helm of an oil tanker. One mode to do this is to measure the combined weight of the tanker and the helm, then have the helm get out the ship and measure out the weight again. The deviation between these two large numbers would be the weight of the helm. If we had a scale that was accurate to many, many significant figures, so nosotros could possibly perform the measurement in this style. Simply you likely realize that this is an impractical way to accurately measure out the weight of the captain and most scales do not have sufficient precision for an authentic measurement. Similarly, trying to measure a small deviation between two large signals of radiation is prone to error since the difference in the signals might exist on the order of the inherent noise in the measurement. Therefore, the degree of error is expected to be high at low concentrations.
The give-and-take higher up suggests that information technology is best to measure out the absorbance somewhere in the range of 0.1 to 0.8. Solutions of college and lower concentrations have higher relative error in the measurement. Low absorbance values (loftier transmittance) correspond to dilute solutions. Often, other than taking steps to concentrate the sample, we are forced to measure samples that have low concentrations and must take the increased fault in the measurement. It is by and large undesirable to record absorbance measurements above 1 for samples. Instead, it is better to dilute such samples and record a value that volition be more precise with less relative error.
Another question that arises is whether it is adequate to utilize a non-linear standard bend. As nosotros observed before, standard curves of absorbance versus concentration will bear witness a non-linearity at college concentrations. Such a non-linear plot can usually exist fit using a higher order equation and the equation may predict the shape of the curve quite accurately. Whether or not it is adequate to utilize the non-linear portion of the bend depends in office on the absorbance value where the non-linearity starts to appear. If the non-linearity occurs at absorbance values higher than 1, it is usually better to dilute the sample into the linear portion of the bend because the absorbance value has a high relative mistake. If the not-linearity occurs at absorbance values lower than 1, using a non-linear college club equation to summate the concentration of the analyte in the unknown may be acceptable.
Ane thing that should never exist washed is to extrapolate a standard curve to higher concentrations. Since non-linearity will occur at some signal, and there is no style of knowing in advance when it will occur, the absorbance of whatever unknown sample must be lower than the absorbance of the highest concentration standard used in the preparation of the standard curve. It is likewise non desirable to extrapolate a standard curve to lower concentrations. At that place are occasions when not-linear furnishings occur at low concentrations. If an unknown has an absorbance that is below that of the lowest concentration standard of the standard curve, it is preferable to fix a lower concentration standard to ensure that the bend is linear over such a concentration region.
Some other concern that always exists when using spectroscopic measurements for compound quantification or identification is the potential presence of matrix effects. The matrix is everything else that is in the sample except for the species being analyzed. A concern tin can occur when the matrix of the unknown sample has components in it that are not in the bare solution and standards. Components of the matrix can have several undesirable effects.
What are some examples of matrix effects and what undesirable result could each have that would compromise the absorbance measurement for a sample with an unknown concentration?
I business organization is that a component of the matrix may absorb radiation at the aforementioned wavelength equally the analyte, giving a simulated positive signal. Particulate matter in a sample will scatter the radiations, thereby reducing the intensity of the radiation at the detector. Scattered radiation volition be confused with absorbed radiation and result in a college concentration than actually occurs in the sample.
Another business is that some species have the power to change the value of \(\lambda\)max. For some species, the value of \(\lambda\)max can show a pronounced dependence on pH. If this is a consideration, then all of the standard and unknown solutions must be accordingly buffered. Species that can hydrogen bail or metal ions that can form donor-acceptor complexes with the analyte may modify the position of \(\lambda\)max. Changes in the solvent can affect \(\lambda\)max likewise.
Beer's Law Y Mx B,
Source: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Molecular_and_Atomic_Spectroscopy_%28Wenzel%29/1:_General_Background_on_Molecular_Spectroscopy/1.2:_Beers_Law
Posted by: richardssichiple.blogspot.com


0 Response to "Beer's Law Y Mx B"
Post a Comment